Long-Acting Ocular Implant
Prediction of Long-acting Release Profile for Ocular Implant and Mechanistic Understanding of Performance

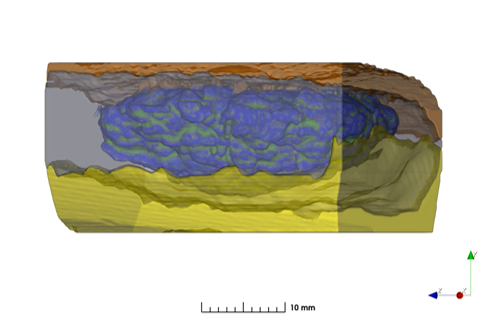
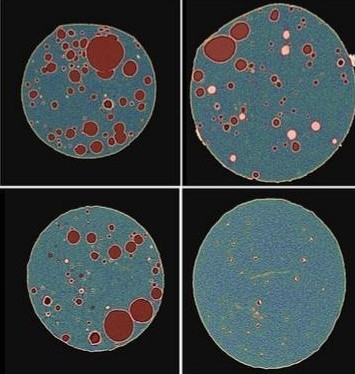
Long-acting implants and injectables are typically formulated using carrier(s) with specific physical and chemical properties, along with the active pharmaceutical ingredient (API), to achieve the desired daily exposure for the target duration of action. In characterizing such formulations, real-time in-vitro and in-vivo experiments are lengthy, costly, and labor intensive as these implants are designed to last for 6-12 months or longer. A novel characterization technique, combining high resolution three-dimensional X-Ray microscopy imaging, image-based quantification, and image-based release simulation, has been employed to provide a mechanistic understanding of formulation and process impact on the microstructures and performance of a PLGA polymer implant.

Artificial intelligence-based image segmentation and image data analytics were used to convert morphological features visualized at high resolution into numerical microstructure models. These digital models can be used to calculate key physical parameters governing drug transport in the polymer matrix, including API uniformity, API domain size, and permeability. This powerful new tool has the potential to advance the mechanistic understanding of interplay between drug microstructures and performance, and accelerate the development of robust drug eluting long-acting implants and controlled release formulations.
Additional Case Studies
Our Expertise
In Numbers
Programs Supported
Formulations Digitized
Pharmaceutical Partners

Transform Your Program with Microstructure Science
Get started with a drug product digital twin.