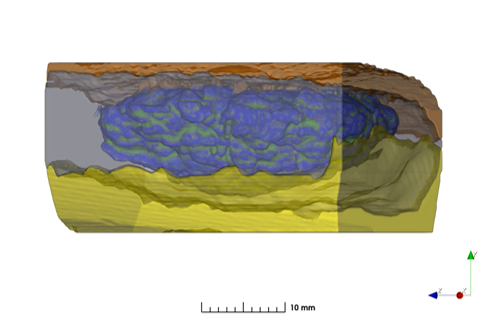
Impact of PEG Grade on Performance of Dexamethasone Ophthalmic Inserts

In this publication from the FDA, the impact of PEG-SG source on product performance was evaluated. This research is important for generic development of products like Dextenza, which use PEG as a funcitonal excipient for hydrogel formation. In this paper, FDA officers demonstrated the impact of crosslinking density on product quality across different formulations, applying X-ray microscopy imaging and microstructure analysis with the digiM I2S software.
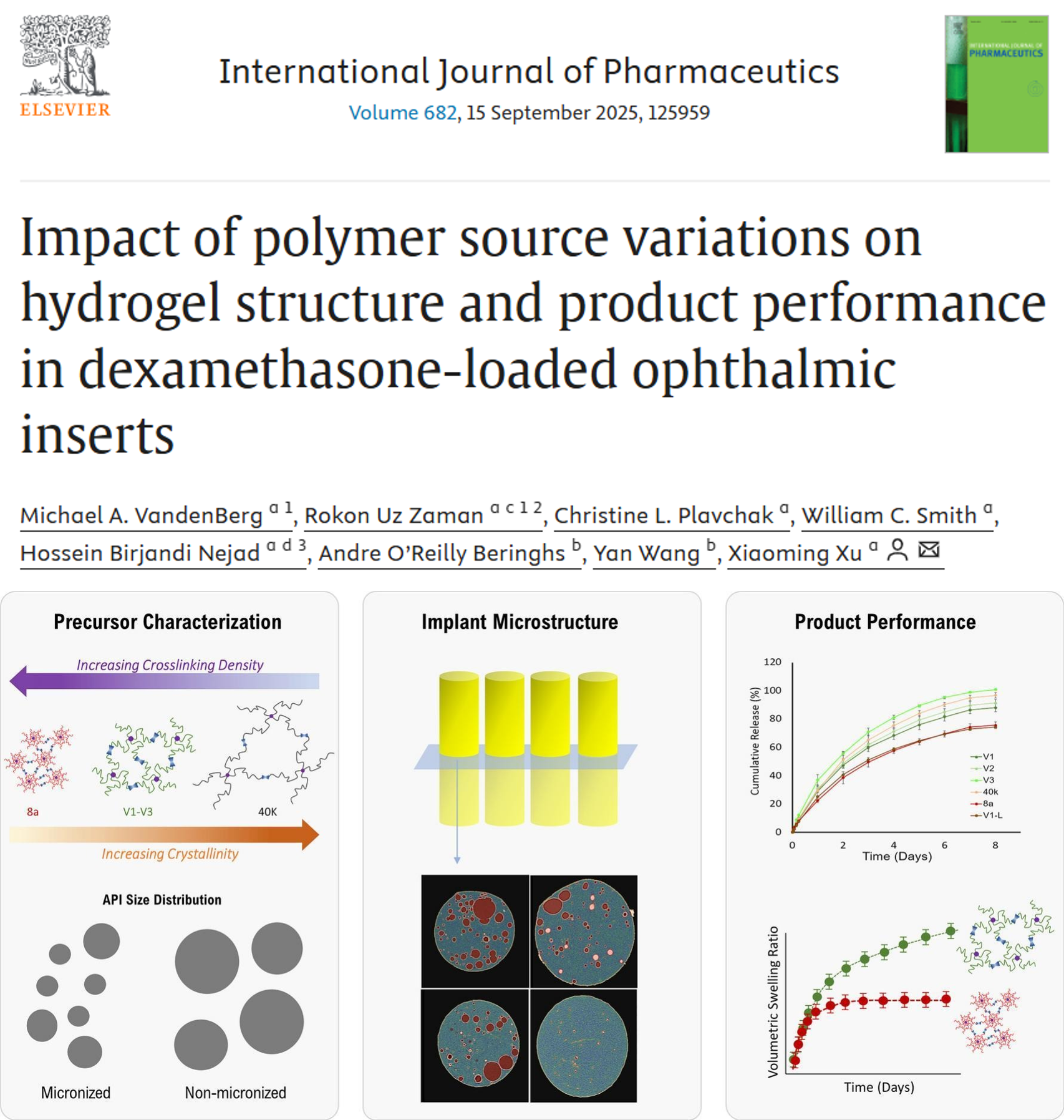
Working via digiM's contract with the FDA Office of Pharmaceutical Quality, digiM and FDA scientists applied the I2S platform for image analysis and microstructure characterization. In the study, porosity and pore size of formulations was found to be higher in formulations with higher molecular weight or increases in branching. Dexamethasone API particle size distribution in the inserts were also measured and found to agree agree with anticpated differences between batches with micronized and non-micronized API sources.
This case study is an important milestone in digiM's relationship with the FDA, with I2S software technologies demonstrated as a critical tool in regulatory research and acceleration of generic drug product development.
Additional Case Studies
Our Expertise
In Numbers
Programs Supported
Formulations Digitized
Pharmaceutical Partners

Transform Your Program with Microstructure Science
Get started with a drug product digital twin.