Spray Dried Dispersions
Visualize and control particle microstructure to improve dissolution, stability, and manufacturability at every stage of development.

Who this is for
Drug product and process development teams working on spray‑dried dispersions (SDDs) for tablets, capsules, granules, and DPIs, especially when scaling across sites or CDMOs.
The Problem
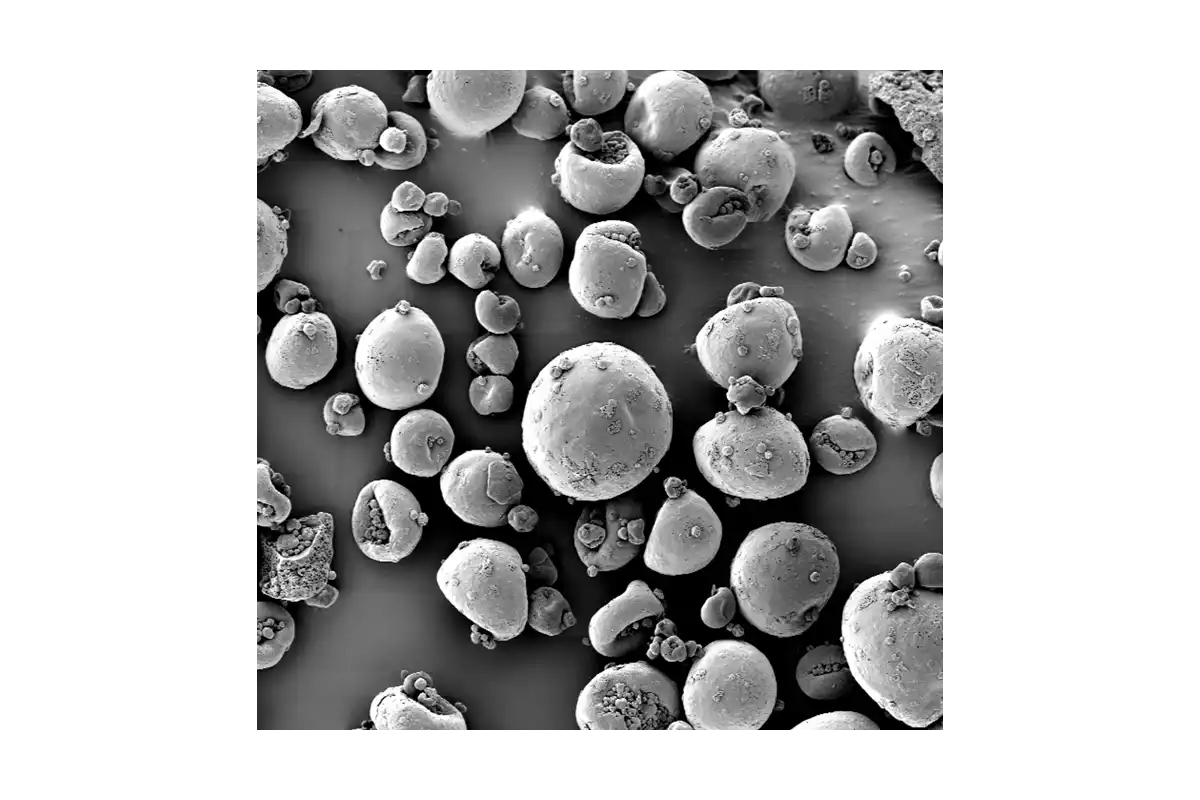
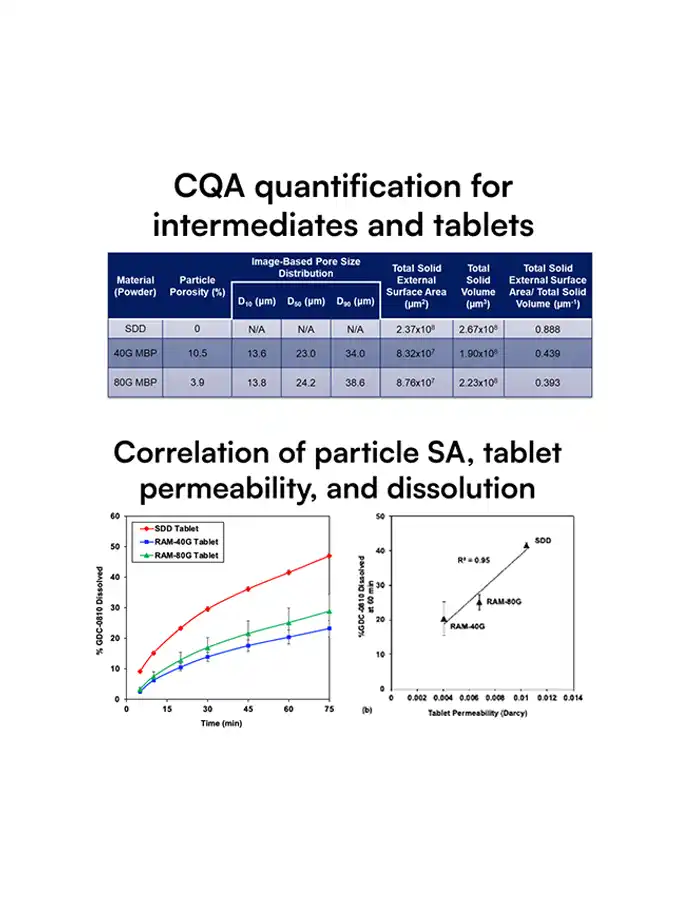
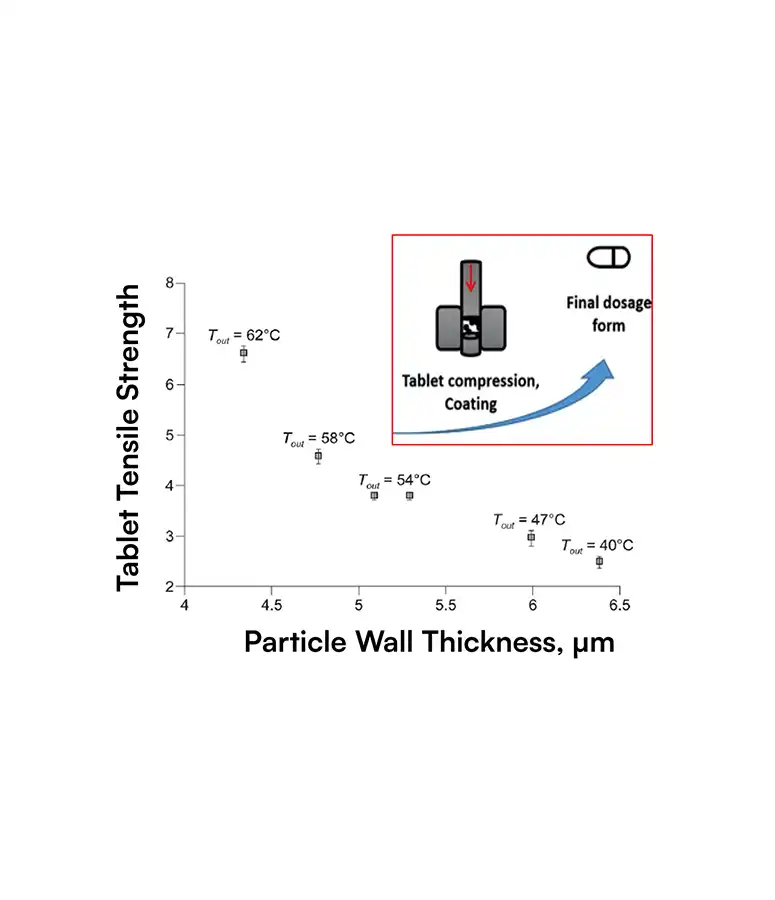
SDD particles have complex, hollow morphologies that strongly influence dissolution, stability, tablet strength, and ultimately bioavailability and yield.
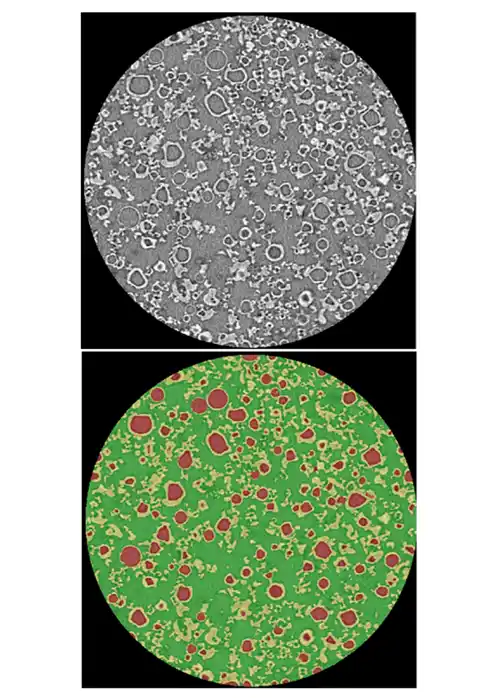
Traditional tools cannot quantify these structures at scale, so teams only see the business impact as variability: failed dissolution tests, tablet defects, site‑to‑site differences, clinical surprises.
Business Outcomes
Our Solution: SDD Microstructure Insight Sprint
A defined 3 to 6 week engagment that combines 3D X-ray micro CT imaging and advanced AI analytics to:

Heading 1
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
- Item 1
- Item 2
- Item 3
Unordered list
- Item A
- Item B
- Item C
Bold text
Emphasis
Superscript
Subscript

Heading 1
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
- Item 1
- Item 2
- Item 3
Unordered list
- Item A
- Item B
- Item C
Bold text
Emphasis
Superscript
Subscript

Heading 1
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
- Item 1
- Item 2
- Item 3
Unordered list
- Item A
- Item B
- Item C
Bold text
Emphasis
Superscript
Subscript
What You Get
Formulations
Studied

Transform Your Program with Microstructure Science
Get started with a drug product digital twin.