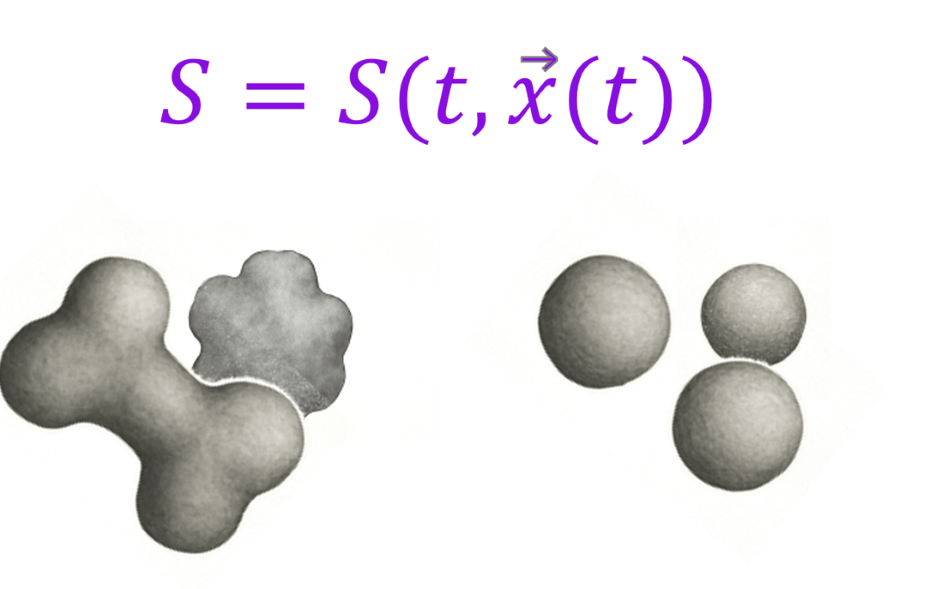Seeing is Believing, Especially for Dissolution
In situ imaging to capture tablet dissolution in real time, plus microstructure predictive release modeling

It took me almost 3 years.
3 years to do what you ask? I’ll start from the beginning. In September 2022, about 2 weeks after I started at digiM, learning about all of the cool things digiM did, starting from imaging, continuing to machine learning image analysis and advanced characterization of various dosage forms, I had the need to pose a question to our technical team - why are we not doing anything with imaging of dissolution?
What do you mean? They said, and at that point I went on a rant that we are missing out on one of the most fascinating, yet most challenging areas in pharmaceutical development. It’s not that the team did not know what dissolution is, they just did not realize how critical and fundamental dissolution is to so many of the scientists we talk to on a regular basis.
For full disclosure, I did not know much about dissolution when I started my career in sales. I got to know more about it selling Pion fiber optic systems for a living. It was quite successful because it helped alleviate significant bottlenecks in dissolution. Robustness, repeatability, accuracy, and time spent in the lab, to name a few. All of you that are familiar know exactly what I’m talking about. And I’m not even talking about dissolution and how it can be (though often fails) as a predictive test to know if a certain drug will reach its desired PK profile. This alone adds a few levels of complexity that scientists are trying to address for years now.
Writing this, I had to remind myself how far back in history dissolution goes. Here are a few fun facts:
- The Noyes-Whitney equation we all know goes back to 1897.
- In 1971 the basket-stirred flask test (USP Apparatus 1) was adopted as an official dissolution test in six monographs
- In 1978 the paddle method (USP Apparatus 2) was introduced
- We have to go back almost 30 years to 1997 where FDA published 4 guidelines on in vitro release
So, if dissolution science tracks decades back, why is there nothing out there on imaging? Well, here we have to be careful about how we categorize imaging. In our context, when we at digiM talk about imaging, we are referring to physical microscopy imaging of either the drug substance, drug product and anything in between.
Spectroscopic imaging has been around for a long time and has been instrumental in gaining important information on the performance of the drug. Raman and near IR were proven to be ideal for certain applications like elemental composition, analysis as one example.
So going back to my question about imaging and dissolution. To be specific, I asked if it's possible to image a tablet as it dissolves. The answer I got is that there are limitations in time-resolution and hardware to visualize dissolution in the same way 3D manner that we typically perform for dry samples.
Well, 3 years after it appears the gap is getting smaller on two fronts.
First, using in situ imaging on synchrotron beamlines, together with careful design of a sample holder to keep the tablet still, we are now able to image a tablet in 3D as it dissolves. Immediate release and modified release are all on the table to scan and extract critical information on formulations, as an example – coating erosion, porosity development, swelling of polymers, disintegration, and seeing the ingredient fragments involved in actual dissolution. All highly quantitative parameters, critical to performance understanding, which are otherwise unattainable…

Secondly, and even more exciting (I know it's hard to imagine such a thing exists after looking at these images..!) is the ability to predict dissolution, without exposing it to any media or compendial USP apparatus. Using real, what we call ground truth microstructure data, with SEM or micro-CT images we are now able to predict dissolution profile of ASDs, intermediates, granules, tablets etc. Is it perfect? No, and there are still things to improve of course. I will not claim that we have eliminated completely the need to perform a dissolution test, but at the very least, we narrowed the design space, and hopefully, made it possible for scientists to spend less time in the lab running samples when they are scarce in quantity, consuming media and time, and have real questions on how particle morphology plays a role in design the optimal formulation and making sure the asset in play is manufactured in the optimal way.
How did our team do it? Great question, and for that we can provide numerous resources for review.
Synchrotron micro-CT imaging: “You mustn’t be afraid to dream a little bigger, darling”
So, after 3 years, I can now say that yes, imaging and dissolution is something worth exploring. I don’t know what the future holds, but I can definitely say the next 3 years will be just as exciting!
Transform Your Program with Microstructure Science
Get started with a drug product digital twin.
















.jpg)