De-risking the “Penalty of Change” in Drug Product Development and Manufacturing
Use of advanced microscopy and microstructure characterization for equivalence by design

Earlier in my pharmaceutical career, a wise mentor remarked that “an unexpected finding in drug discovery can be a welcome opportunity, where as a surprise in late product development is often seen as a potential disaster.” These sharply contrasting views highlight how flexibility to change differs across the discovery, development and manufacturing continuum. It underscores the rising cost of change in terms of resources, timeline risk and potential negative impact to CMC regulatory review as a drug product advances in development and manufacturing.
Another way to describe the cost of flexibility is the “penalty of change” (PoC), to which my former Merck & Co. colleagues nicely illustrate in a detailed perspective paper. While PoC may not be a common term in pharmaceutical R&D, it is certainly one that resonates to those who have experienced it and is a useful construct in devising strategy to increase flexibility with lower penalty during drug product development.
Here’s a simple truth: changes are inevitable during drug development-- API attributes, the type and composition of excipients, manufacturing processes and equipment, process scale-up and transfer to commercial sites – are just some of the many nodes of change that occur. And when a change does happen, the question is: what is the impact on drug product performance and quality? Of course, the impact of change can be interdependent and cumulative. It is often magnified in later stages of development and especially when manufacturing complex dosage forms. So, the money question becomes: how does one mitigate risk and decrease the PoC?
The utilization of advanced characterization and measurement techniques are essential tools to understand the impact of changes to material properties and process parameters on product quality attributes and performance. Importantly, advanced image-based characterization and analysis of the nano- and microstructures that underpin drug product performance can unveil critical attributes not readily captured by traditional analytical techniques. While it’s easy to say, “let’s do the CT scan ,” the advanced image processing and scientific expertise needed to quantify and interpret the microstructures of various drug product dosage forms can be a formidable undertaking.
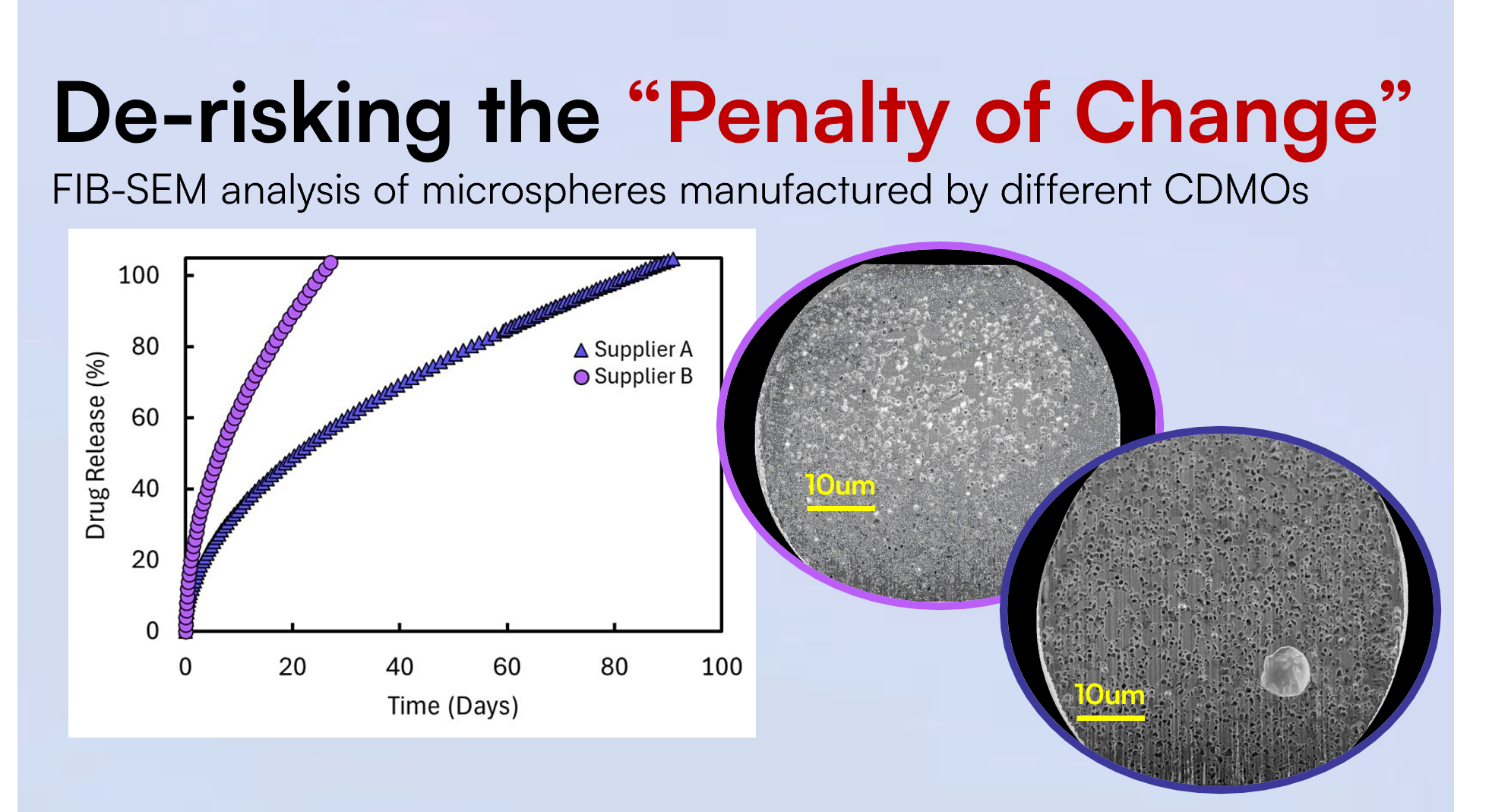
Fortunately, digiM Solution provides a suite of industry-leading microscopy and image analysis technologies that deliver deep microstructure insights to predict and optimize drug product quality and performance. Some of these techniques include 3D X-ray microscopy, FIB-SEM, and mosaic-SEM. When combined with AI image analysis and proprietary software solutions, predictive simulations can be generated from images to reveal the role of drug product microstructure attributes to in vitro dissolution and drug release profiles of complex dosage forms.
High-resolution microstructure imaging and simulations to assess and predict the impact of material properties and process parameters on product quality can inform microstructure-related CQAs in support of regulatory assessment. This offers significant value to both innovators and generics who apply an equivalence-by-design approach in development that integrates a microstructure feedback loop to de-risk and lower the “penalty of change.”
A common de-risking effort is amorphous solid dispersions, where digiM's solution has been routinely used to define sources of dissolution variability and compaction differences across scale up batches. Long-acting injectables are another complex dosage form where de-risking the impact of development changes have been realized, with an emphasis on performance prediction and understanding of product attributes within the injection environment.
Changes during drug product development are going to happen. Whether the goal is increasing flexibility while lowering the PoC from early to late product development, or supporting the construction of data packages for SUPAC, ANDA and NDA filings, digiM’s advanced microstructure imaging analysis can significantly help de-risk the PoC to accelerate your pharmaceutical product development and regulatory success.
Transform Your Program with Microstructure Science
Get started with a drug product digital twin.



.jpg)