Innovator Drug Development
Revolutionizing drug product development with advanced microstructural analysis & Quality by Design approach to accelerate timelines & enhance quality.

Issue
Drug product formulation and design often relies on previously defined sets of ingredients and what formulations have worked before. In order to meet clinical deadlines, the formulation design can often be rushed, and the amount of resources spent in understanding the formulation is limited. As drug product manufacturing is scaled and processing evolves, variability in performance and quality can arise. These challenges can be addressed through a closer understanding of ingredient physicochemistry during the formulation stage, derisking variability upon scale up and downstream manufacturing changes.
Common Challenges
- Downstream manufacturability and quality issues rooted in oversimplified formulation strategies
- Dissolution variability during scale up and technology transfer
- Long development times and resources spent optimizing manufacturing
- Lack of discriminatory in vitro methods to guide formulation and anticipate in vivo performance
Solution
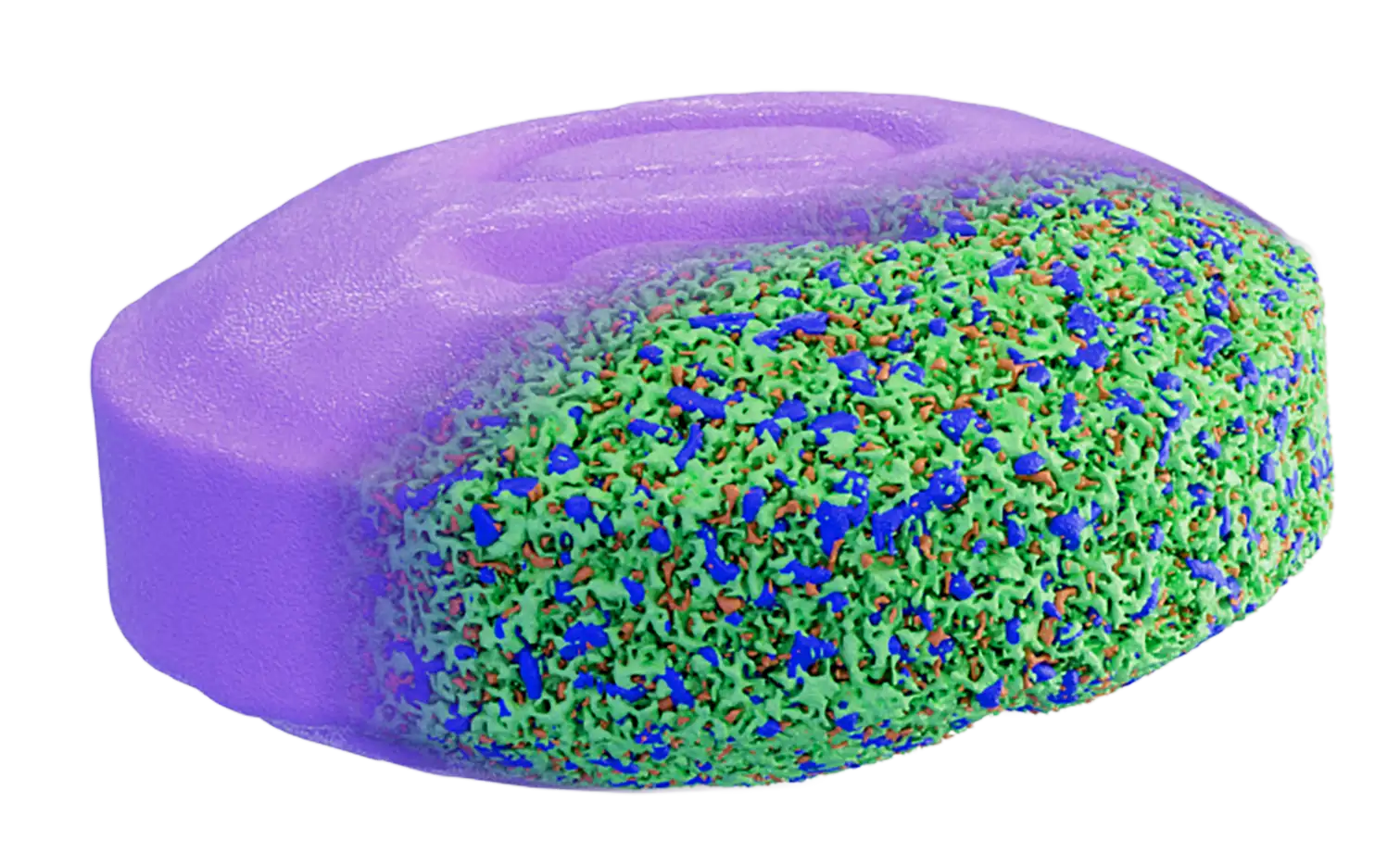
Our mission is to transform the approaches around drug product development through a deeper assessment of ingredient microstructural interactions. To help our clients overcome the long lifecycle of product development, we apply a quality by design approach integrating a microstructure feedback loop. We use advanced microscopic analysis such as FIB-SEM and X-ray micro-CT to quantify the size, distribution, and interaction of ingredients and porosity within the final drug product. Microstructure digitization provides dozens of critical quality attributes to evaluate product performance and quality, serving as a data rich input for QbD integration. For controlled release platforms, we predict release profiles in a matter of days, using the real digitized microstructures to simulate transport phenomena. This can eliminate the need for long in vitro tests during formulation and process selection stages. We also study post-partial release samples from in vivo and in vitro studies, enabling quantification of the drug dissolution front, polymer degradation, and other structural changes that can occur. This analysis can offer insights into the impact of the in vivo environment on release and can support the pharmacokinetic data.
In addition to our analysis services, we support the assembly of simulated release profiles and microstructure analysis into data packages for NDA filings.

Our Approach
- Guide formulation design through quantitative analysis of ingredient interactions
- De-risk scale up and tech transfer challenges with structural benchmarks
- Build correlative process models to identify critical process parameters and critical quality attributes
- Predict dissolution, disintegration, and release phenomena from microstructure digital twins
Transform Your Program with Microstructure Science
Get started with a drug product digital twin.